Head of Research Program: Vladimír Kryštof
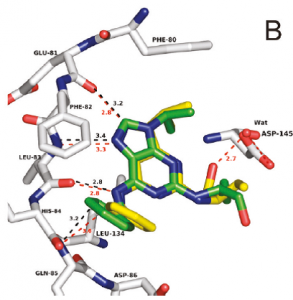
Overlay of roscovitine and its pyrazolopyrimidine isostere within the active site of CDK2. Adapted from J. Med. Chem. 2011, 54, 2980–2993.
Changes in protein phosphorylation are known to be important in almost all cellular pathways including those that regulate proliferation and the cell cycle. Cyclin-dependent kinases (CDKs) are enzymes that play key roles in the control of cell cycle entry (CDK4 and CDK6), DNA replication (CDK2), and the initiation of mitosis (CDK1). Some CDKs also have roles beyond the cell cycle in processes such as basal mRNA transcription, splicing, the maintenance of neuronal function, apoptosis, motility, stem cell self-renewal and spermatogenesis.
Numerous abnormalities related to CDKs have been identified in cancers, including for example mutation or silencing of the gene encoding p16INK4A, mutation of RB1 or its inactivation at the protein level, deregulation of D-type cyclins, amplification of the CDK4 gene, or amplifications of E-type cyclins. These molecular changes are very well known and their identification has prompted the development of small molecule CDK inhibitors that block cellular proliferation and induce cell death, both as tools for cell biologists and experimental oncologists.
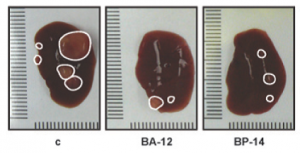
Representative morphologies of engodenous liver cancer in mice (control) and those treated with two CDK inhibitors. Adapted from Mol Cancer Ther 2013;12:1947-1957.
The gradual increase in understanding of the functions of CDKs in normal and transformed cells was accompanied by the initiation of multiple development programs using both structure- and ligand-based approaches that have yielded a range of small molecule CDK inhibitors, many of which have since entered clinical trials, including (R)-roscovitine (Seliciclib, CYC202), originally developed by our group. We have also prepared and characterized other CDK inhibitors based on purine scaffolds and their bioisosteric alternatives such as 8-azapurines, pyrazolo[4,3-d]pyrimidines or pyrazolo[4,3-e][1,2,4]triazines. Many of the compounds display superior kinase inhibition and anticancer activity both in vitro and in vivo.
Compounds LGR1404 and LGR1406 completely inhibit VEGF-induced vessel formation in the chorioallantoic membrane (CAM) assay. Adapted from PLoS ONE 2013; 8(1): e54607. In addition, we have recently CDK5 as novel alternative and pharmacologically accessible target in the context of angiogenesis. Our compounds inhibited endothelial cell proliferation, cell migration, chemotaxis and tube formation at the non-toxic concentrations. Furthermore, the compounds reduced mouse aortic ring sprouting, angiogenesis in the chick chorioallantoic membrane, and neovessel formation in the mouse cornea. The phenotype of the migrating cells resembles the previously described effects of silencing of CDK5 in endothelial cells.
Recently, we developed novel derivatives with potent FLT3 inhibitory activity. FLT3 tyrosine kinase is a potential drug target in acute myeloid leukemia (AML) because patients with FLT3-ITD mutations respond poorly to standard cytotoxic agents and there is a clear link between the disease and the oncogenic properties of FLT3. The lead compound displays nanomolar activity in biochemical assays and selectively blocks proliferation of AML cell lines harboring FLT3-ITD mutations, whereas other transformed and normal human cells are several orders of magnitude less sensitive. A significant therapeutical effect was observed also in a mouse model of AML. Additionally, a single dose of lead compound in mice with subcutaneous MV4-11 xenografts caused sustained inhibition of FLT3 and STAT5 phosphorylation over 48 h, in contrast to the shorter effect observed after administration of the reference FLT3 inhibitor quizartinib.We are convinced that our kinase inhibitors are highly attractive anticancer compounds, with strong and selective activities in cancer cells. The discovery and characterization of kinase inhibitors based on new underexplored scaffolds is a major goal of our current research.
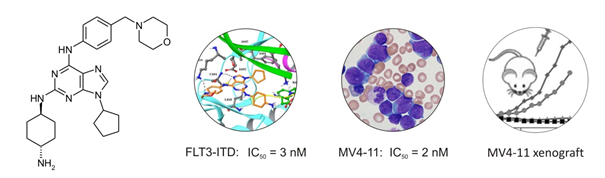 |
| Lead compound displays nanomolar potency in biochemical and cellular assays and demonstrates significant effect in vivo. Adapted from J Med Chem. 2018 May 10;61(9):3855-3869. |
 Laboratory of Growth Regulators
Palacký University Olomouc
Laboratory of Growth Regulators
Palacký University Olomouc